Beautiful! Top Orphan Drug Companies
The companies with the. Top 10 Global Orphan Drug Companies Ranking 2017.

Pharmaboardroom Orphan Drugs In Europe A Snapshot
The costs of granting orphan drug status to partial orphan drugs can be substantial Chua says in part because insurance companies are hesitant to exclude orphan drugs from their formularies a list of drugs covered by the insurer.
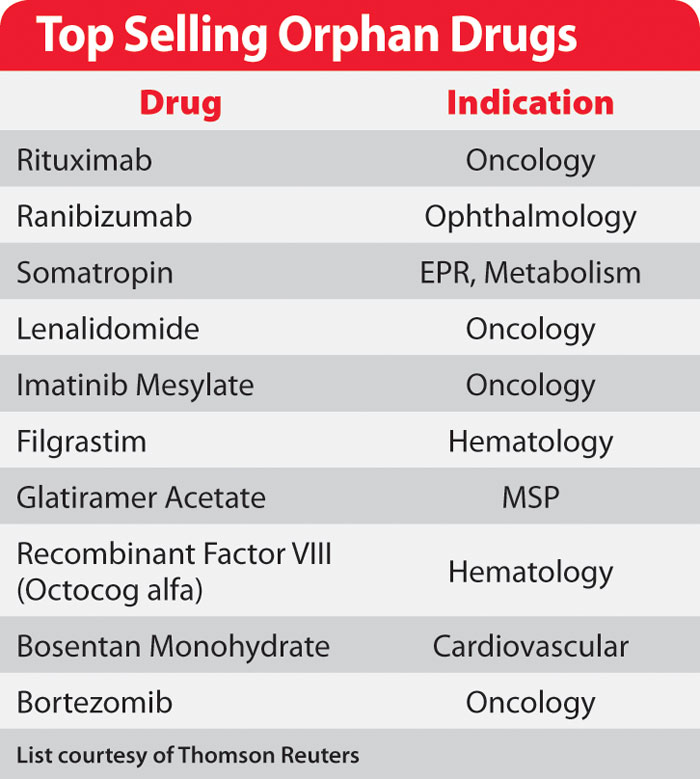
Top orphan drug companies. The FDA is barred from approving any other biologic or small-molecule drug genericfollow-on or otherwise treating the same orphan disease for seven years. Watch this years report into the top performing drugs companies and sales of the orphan market. The FDA Office of Orphan Products Development determines if a drug qualifies as an orphan product.
Growth rates approximately double those of the overall prescription drugs market and the mean cost per patient per year of the top 100 orphan products in the US hitting 150854 in 2018 versus 33654 for a non-orphan drug explain much of the allure. The protection granted to orphan drugs is quite strong. Big pharmas recent dominance of the orphan market has fuelled calls to reform the orphan drug act in the US.
It is projected that by 2024 the top orphan drug by revenue. Add the Michigan Medicine News Break on iTunes Google Podcast or anywhere you listen to podcasts. A manufacturer may at its sole discretion offer discounts on orphan drugs to these hospitals.
The orphan drug application process takes around 6-9 months to complete. A Kaiser Health News investigation calls attention to pharmaceutical companies gaming the Orphan Drug Act to maximize profits. This chart shows the companies which have received the most orphan drug exclusivities in the past five years.
It is a drug used to treat certain types of cancers and genetic disorders. 5 Top Orphan Drug Startups Using our StartUs Insights Platform covering 1116000 startups emerging companies we looked at innovation in the drug discovery field. Made with Visme Infographic Maker.
On orphan drug products demonstrates the promise these companies see in the orphan drug space. The research also notes that MA deals have been the primary way in which big pharma companies secure these orphan drug designations with very few rare disease drugs being developed in-house. Cyclerion Therapeutics is a clinical-stage biopharmaceutical company harnessing the power of soluble guanylate cyclase sGC pharmacology to discover develop and commercialize breakthrough.
In Korea orphan drugs are supplied to patients by pharmaceutical companies or the Korea Orphan Drug Center. In 2017 125 billion worth of rare disease drugs were sold - a total that. And the global market for orphan drugs targeting rare diseases is growing at double the rate of the non-orphan market.
As of October 2002 over 130 orphan drugs have been approved by the KFDA. The Orphan Drug Designation List was updated and developed using the methodology referenced in About the Orphan Drug List. Regardless orphan drug sales are forecast to increase from 119bn in 2018 to 217bn in 2024.
Made with Visme Infographic Maker. Ad To produce liquid-solid semi-solid dosage forms for oral-nasal-buccal-topical delivery. The Orphan Drug Act was passed in 1983 to give drug companies incentives to develop treatments for rare diseases.
Orphan Drug Designation List. The top 10 companies in the world ranked by global orphan drug sales and market share in 2017. As reported in NewsWise.
Around 30 million Americans suffer from 7000 or so rare diseases. Celgene takes top spot followed by Roche and Novartis. Its not hard to see the allure of orphan-drug development.
The top orphan drug based on revenue in 2018 was Revlimid. Celgene the company that makes Revlimid made more than 184000 in revenue for each patient who takes this drug. Companies finding solutions for rare diseases face less competition if any at all from branded.
Drug companies have demonstrated an increasingly common pattern of gaming the system by slicing and dicing indications so that drugs qualify for lucrative orphan status benefits and getting. It should be used to govern the quarter October 1 to December 31 2021. The top-selling orphan drug in the United States in 2017 was Revlimid with 5 billion in sales.
Government started the orphan drug program 1983 designed as a financial and regulatory boost to make the development of new treatments for. A new research note from Credit Suisse acknowledges five top biotech companies that are filling the orphan multi-drug multi-disease space in a big way. Top 20 orphan drugs by 2018.
Top 10 Rare Diseases Orphan Drugs Novartis Roche Celgene Rankings. Rare drugs are few and far between with a cliental of less than 200000 Americans dissuading drug companies from taking on the enormous charge of developing novel treatmentsIn an effort to propel rare progress the US. EvaluatePharma Orphan Drug Report 2020.
The Orphan Drug Landscape in 2024 This rising market growth continues to fuel calls to reform the Orphan Drug Act in the US from those who argue that big pharma should not be benefiting from the regulatory and tax benefits meant for orphan drug developers. For this research we identified 231 relevant solutions and picked 5 to showcase below. Biopharmaceutical companies are attracted to the orphan-drug market because it can be very lucrative.
Global Rankings Rare Diseases. Top Rare diseases companies globally. Big pharma meanwhile is forecast to make up 8 of the top 10 orphan drug companies in 2024.
Eurofins CDMO presents global offerings for the development of high-potency drugs. Top 10 companies by global revenue from orphan drugs 2024 Market share of top 20 companies for orphan drugs worldwide 2018 and 2024 US and European private VC-backed orphan. Swiss giants Novartis and Roche take the top two places followed by American firm Celgene.
The Top 10 rare disease companies globally ranked by worldwide orphan drug sales for 2018. Top Orphan drug Start-ups Top ranked companies founded since 2016 for keyword search. The Orphan medicinal products regulation of the European Parliament and the United States Orphan Drug Act aim to incentivise pharmaceutical companies to develop medicines for rare diseases that would otherwise not be commercially viable A measure of the success of these regulations is the number of orphan drugs approved.
GARD has information from the Food and Drug Administration FDA on treatments approved for rare diseases known as orphan productsdrugs.

Pharmaboardroom Orphan Drugs In The Usa A Snapshot

Top Orphan Drug Companies By Market Share 2024 Statista

The New Economics Of Orphan Diseases
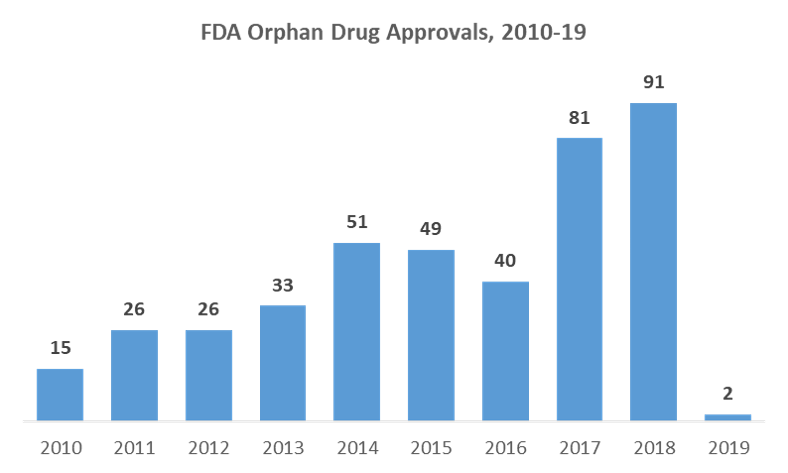
Orphan Drugs Commercializing Rare Disease Drugs Kx Advisors
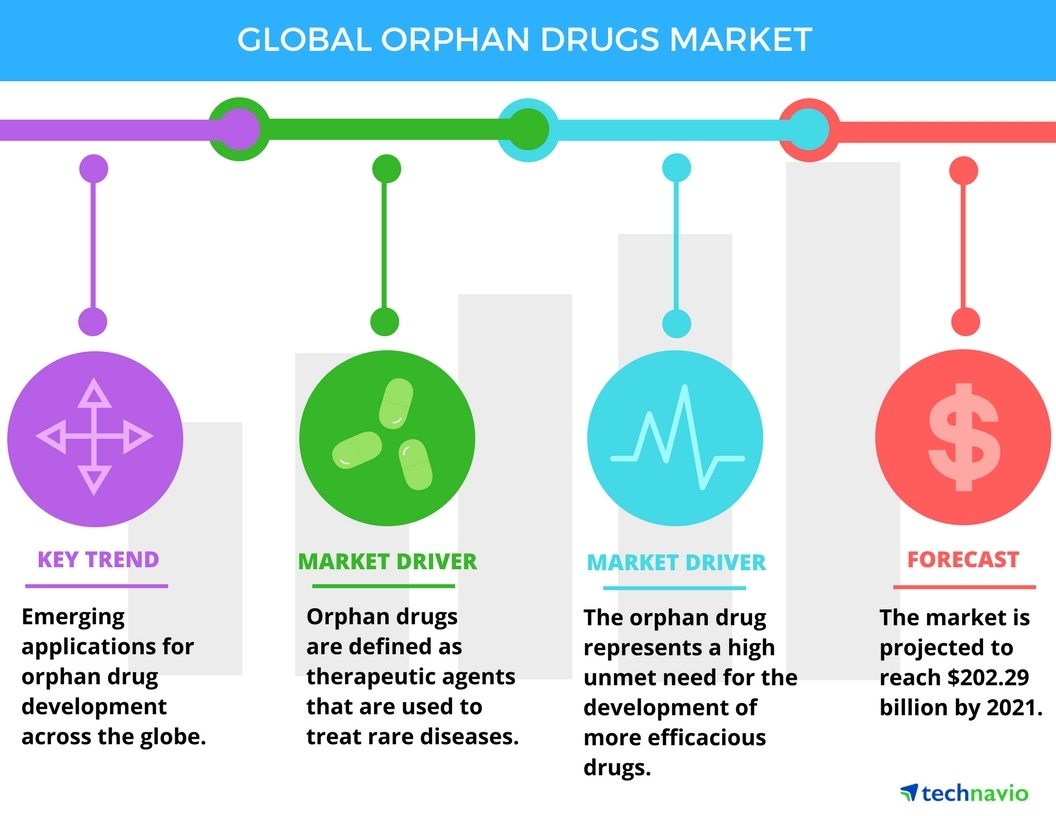
Global Orphan Drugs Market Drivers And Forecast From Technavio Business Wire

Orphan Drugs In 2017 An Analysis Of Clinical Trials

Modifying The Criteria For Granting Orphan Drug Market Exclusivity Value In Health

Orphan Drugs Face Uphill Battle In 2020

Top 10 Pharma Companies In Selected Asia Pacific Markets Q4 2011 Sales Pharma Companies Pharma Marketing

Global Orphan Drugs Market Assessment Industry Analysis Clinical Trial Pipeline Analysis And Revenue Forecast Till 2028 Medgadget

Top Selling Global Orphan Drug By Revenue Forecast 2024 Statista
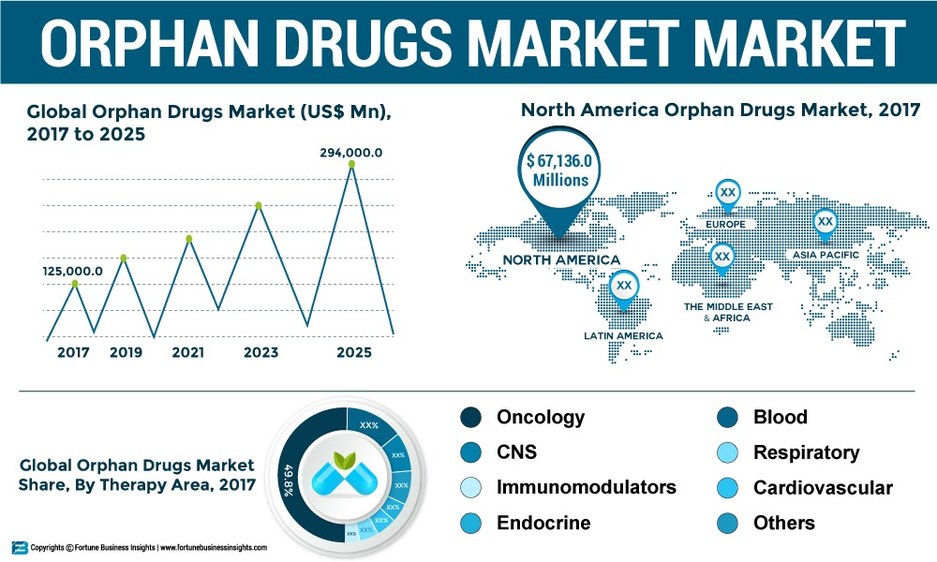
Orphan Drugs Market To Reach Us 294000 Mn By 2025 Increasing Development Of Oncology Related Orphan Drugs To Drive The Market Fortune Business Insights

Top Orphan Drug Companies By Market Share 2024 Statista
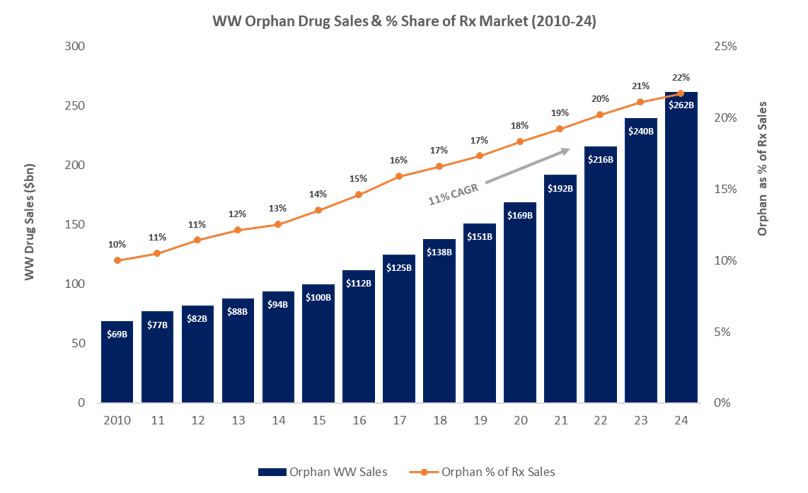
Orphan Drugs Commercializing Rare Disease Drugs Kx Advisors
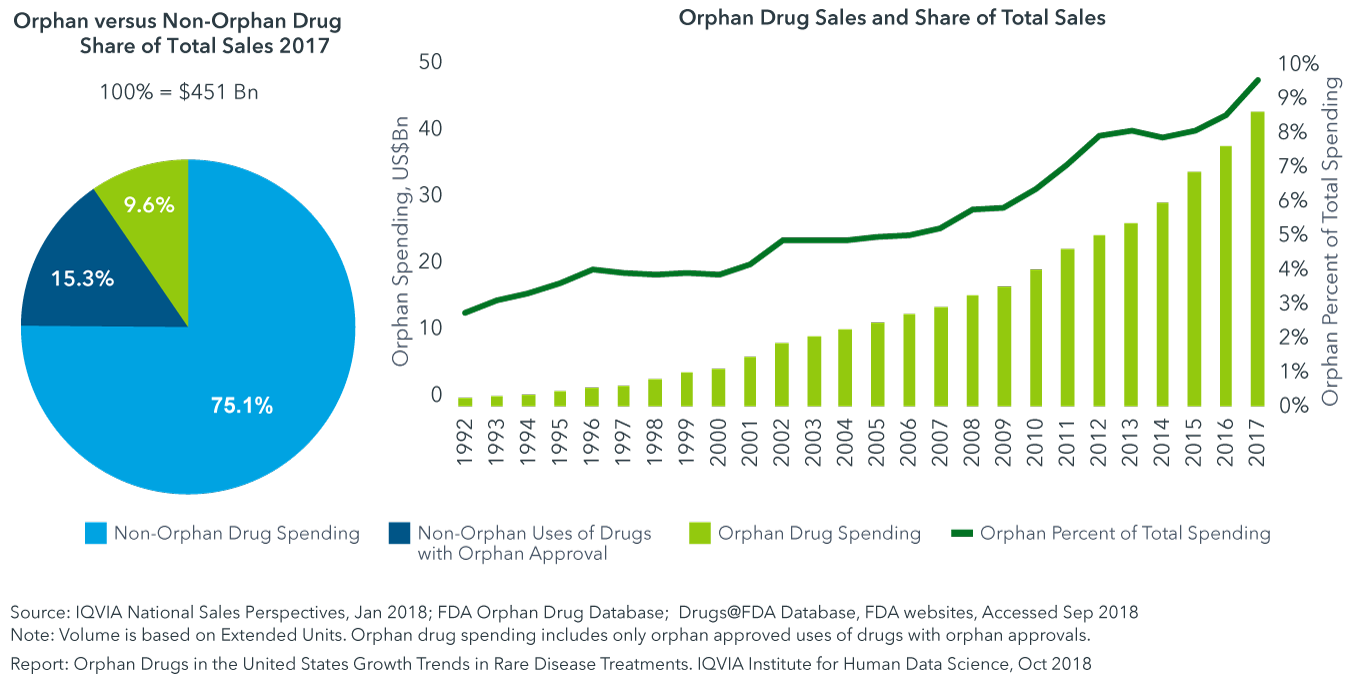
Top 4 Criteria For Choosing The Right Cdmo For An Orphan Drug Regis Technologies



